|
Understanding Acidic Rain and Its Impact on Rainwater Harvesting
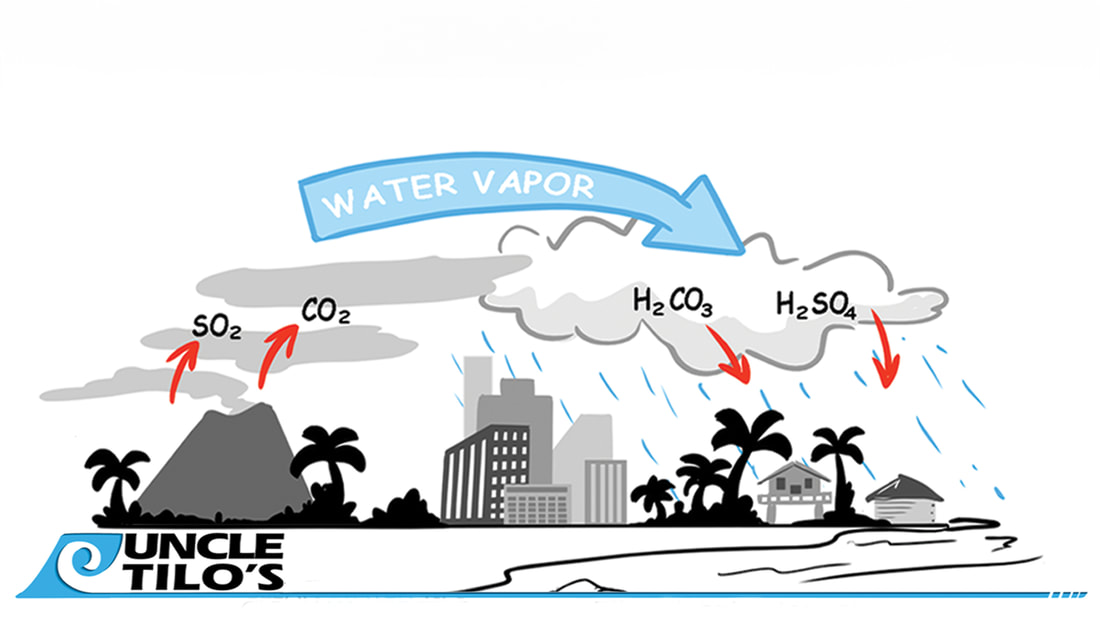
As a rainwater harvester, it's crucial to understand the causes of acidic rain and how to protect your system from lead contamination. In areas like Hawai'i, rainwater can have a pH as low as 4 due to volcanic emissions, notably from Kilauea Volcano. This acid rain, more acidic than the typical pH 5.6 due to atmospheric CO2, poses significant risks to catchment systems. The Dangers of Metal Corrosion and Lead Contamination Acidic rainwater can corrode metal components in your catchment system, especially those made of iron, copper, and brass. This corrosion increases the risk of lead leaching if these metals are present in galvanized and brass fixtures. Lead in drinking water is a severe health hazard known to cause cognitive and developmental issues, particularly in children. Strategies for Neutralizing Acidic Rainwater To safeguard against these risks, it's essential to neutralize the acidity of collected rainwater. Keeping the pH above 6.5 is critical. This can be achieved through regular maintenance practices like adding baking soda to the catchment tank. For a more consistent solution, products like Uncle Tilo's Acid Rain Mineral Pack offer a slow, continuous release of Calcite and Corosex, which neutralize the water and protect your system's longevity. Proactive Measures for Safe and Reliable Rainwater Harvesting By understanding the causes of acidic rain and taking proactive steps to neutralize pH levels, rainwater harvesters can effectively minimize the risk of lead contamination, ensuring the safety and reliability of their water supply. Remember, the quality of your water directly impacts your health, making this knowledge and these practices invaluable for every harvester.
0 Comments
Leave a Reply. |